SOIL CHROMATOGRAPHY
Splitting Soil: Using soil chromatography to document soil quality in London
2019

This research project focused on soil analysis and how vital soil is as a natural resource for human health and ecosystems. Soil is an incredible component of our planet and soil health often does not receive as much attention as air or water. Soil pollution is essential to monitor due to the increasing soil contamination levels occuring as a result of human activities, from agricultural runoff, city runoff, erosion, mining, landfill, and much more.
According to the Environmental Pollution Center, soil pollution can be defined as: ‘the presence of toxic chemicals (pollutants or contaminants) in soil in high enough concentrations to pose a risk to human health and/or the ecosystem.’ Splitting Soil aims to visually represent our data on soil contamination across London, illustrating our soil sampling techniques and research on the causes and effects of soil pollution. We later explored existing methods and techniques of soil remediation such as bioremediation (microbes) and phytoremediation (plants).
1. RESEARCH, SOIL COLLECTION and DOCUMENTATION PROCESS
Each pair visited a different borough of London and collected two soil samples with the aim of finding contrasting soil quality, i.e. one contaminated sample and one healthy sample. Our designated Borough was Hillingdon.
2. ORGANIC MATTER EXTRACTION
Sodium Hydroxide solution is alkaline and was used in this experiment to extract the organic matter in the soil samples, creating a solution containing the soil’s dissolved elements. Depending on the quality of the soil, this dissolved soil solution will contain different compounds, including nitrogen and sulfur containing groups, proteins, enzymes, vitamins and heavy metals.


3. FILTER PAPER PREPARATION WITH SILVER NITRATE (AgNO3)
AgNO3 is used in this experiment to react with the elements found in the soil. AgNO3 develops in the presence of sunlight and so this part of the experiment was carried out in the darkroom. The filter paper was coated in 0.5% AgNO3 solution – this was done by creating a wick in the center of the paper, then pouring the solution into a petri dish with the wick in contact. The AgNO3 solution seeps into the paper via capillary action.
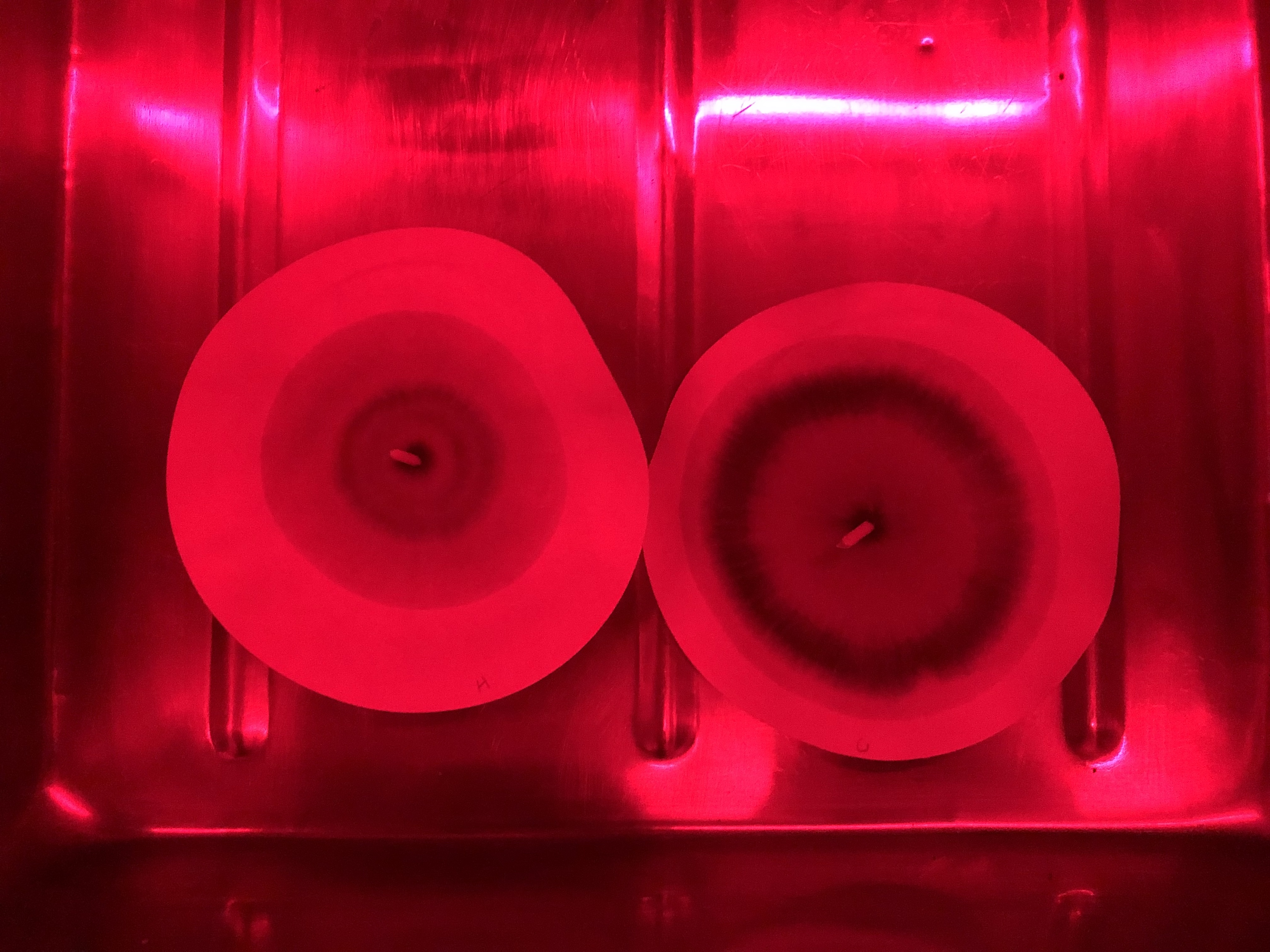

4. RUNNING THE SOIL SOLUTION IN IMPREGNATED PAPER AND DRYING IN INDIRECT LIGHT
Once the coated AgNO3 has been coated with the dissolved NaOH soil solution, the samples are left to dry and subject to indirect sunlight. Upon this light exposure the reacted silver ions on the filter paper will form many different coloured complexes. The colour of these complexes is dependent on the elemental composition of the soil.

Visual Representations of Soil Chromatography & Results


Project conducted in collaboration with Meiqi Peng, MA Biodesign, CSM. Sept 2019
